Exceptional Spirometry products for the medical sector
Our medical air filtration products are manufactured using a range of specific media types which allow them to meet the high performance requirements of the medical market. These medias are fabricated and encapsulated at our manufacturing plant in the UK in order to provide a high quality filtration product capable of meeting the specifications demanded by the NHS and other medical markets.
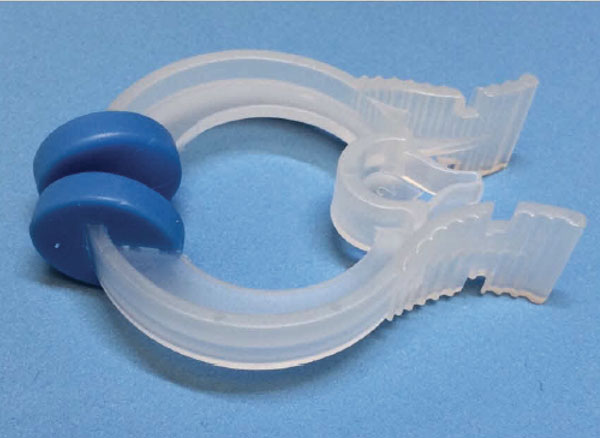
Bacterial /Viral filters
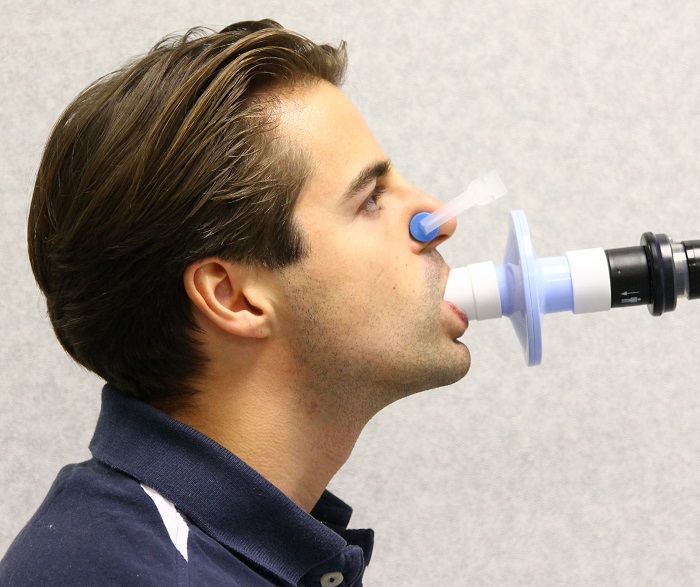
Routine pulmonary function tests may require patients to perform maximal inspiratory and expiratory breathing manoeuvres, and patients may be asked to rebreathe using equipment which may be difficult to disinfect between patients. A subsequent patient carrying out the same manoeuvre on this equipment could inhale infective droplets during the forced inspiratory phase. Very few bacteria are required to facilitate the infectious process of some diseases, such as tuberculosis, and therefore the potential risk of cross-contamination may be high.
Eliminating cross contamination risks
To eliminate the risk of cross-infection, a bacterial /viral filter can be placed between the patients mouth and the pulmonary function test equipment. This is where a single use filter such as the Spiroflo can be used, a single-use filter for all patients overcomes any issues.
As suggested the filters are designed and intended for single patient use, however it is possible for the same patient to use the one filter a number of times during their testing procedure, with the filter being disposed of at the end of the programme in accordance with local infection control procedures.
Multiple equipment use
It is possible that filters can be adapted to fit multiple types of pulmonary function equipment. SpiroFlo offers this adaptability.